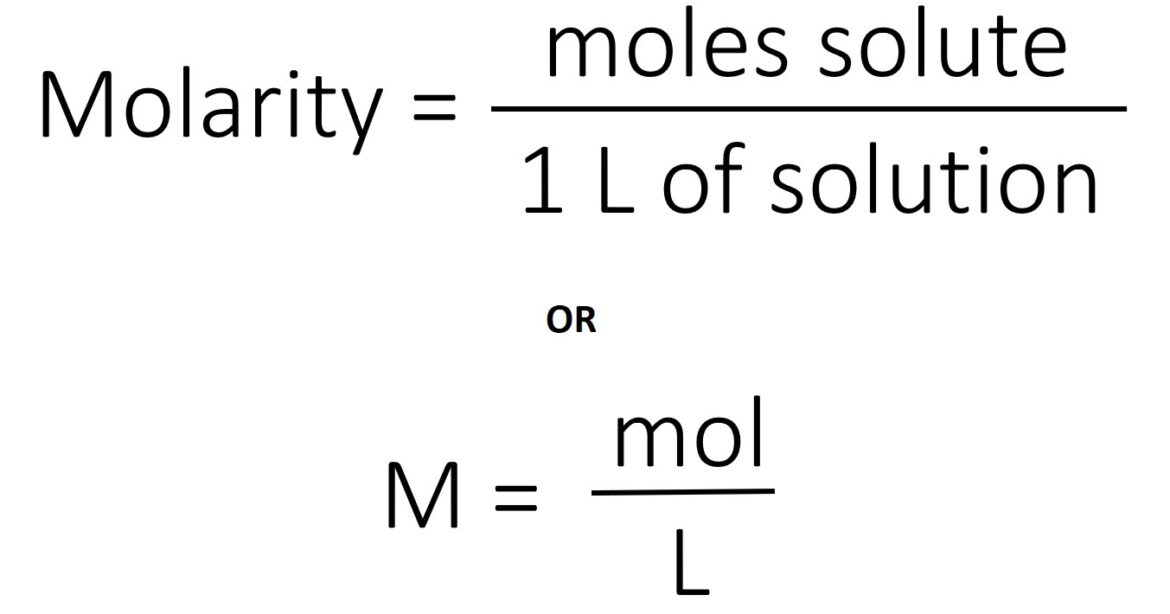There aren’t many things in chemistry as important as molarity. Molarity is the term that describes the exact measurement of a certain concentration in any given mixture. Thanks to molarity we can identify and quantify each compound within any solution. Molarity is calculated using the molarity formula.
Calculating molarity & molarity formula
Molarity is calculated by dividing the number of moles of a solute by the litters of its solution. In the molarity formula, the total volume is measured in liters, whereas the solute is measured in grams.
Molarity= mol solute/L of solution.
Calculating molarity can be also done with the help of a molarity calculator.
All in all, making molar calculations is not that challenging. At least as long as the unit conversions are done right and the formula is applied correctly. That’s pretty much it to get the right molarity. On the other hand, an online molarity calculator makes everything even easier and faster. Plus the margin of error is practically zero.
Mixtures & Solutions
We are literary surrounded by substances that are a mix of various compounds and elements. As a matter of fact, even our bodies are a mixture of all sorts of compounds.
Not many people know that the human body is around 57% water by mass. Basically, we are a mixture of gasses, biological molecules, water, and ions.
If the compounds are mixed and create a uniform composition, then they are named homogeneous solutions. Then there are mixtures whose composition is not even throughout the sample which are called heterogeneous. All solutions can feature components that are gasses, liquids, and/or solids.
From bench to batch
Understanding and calculating molarity is just the beginning of what is to be usually a laboratory synthesis. For example, the synthesis of aspirin includes using two or three grams of salicylic acid. From that, it is expected to get from two to grams of aspirin, conditioned by the used material at the start. This is where molar relationships are used to determine how much product can be made. Of course, anyone with minimum lab experience will tell you that it is practically impossible to get 100% of the theoretical results.
Keep in mind that when producing on an industrial scale, the amount of used materials is way larger. Plus, whenever possible, liquids are preferred since volume can be easily measured which is very important.
But then again, materials such as salicylic acid can be found only in solid form. That means to measure it, it needs to be weighed out.
When a solid material such as salicylic acid is used, it is mixed with reagents for a certain period. The mixing part can take up to several hours and it depends on several factors. That’s a process that is followed by crystallization and purification.
All in All
It all comes down to the experience of the professionals and their experience with molecular synthesis. Then there is their lab and their access to modern apparatus which is essential for producing complex synthetic operations. The point is, for best results, it is super-important to work with someone that has perfected the art of molecular synthesis and for which molarity and molarity calculation is as simple as breathing.